Clinical decision support
The solution screens for plus disease, which is associated with severe ROP.
The new tool measures the diameter of the ventricles of the heart to provide the ratio of the maximum right ventricle diameter compared with the left ventricle. That can help indicate pulmonary embolism severity.
The company will maintain its patent right for the technology.
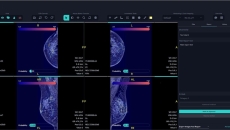
Also, Lunit has received Taiwan's approval for its AI mammography solution.
The AI model still has a modest prediction accuracy and is slated for a wider clinical trial soon.
It will use its fresh funds to complete a multi-site validation study across eight Asia-Pacific markets.
Formerly known as IDx, the company received FDA De Novo clearance in 2018 for its IDx-DR system used to detect diabetic retinopathy.
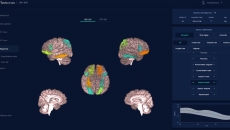
It measures the cortical thickness in a brain scan to provide a patient's level of brain ageing.
Also, a new online repository of health and wellness knowledge has been launched in India.
Health2Sync has upgraded its patient management platform to identify patients' CKD risk levels.





![[Left] Ed Deng, co-founder and CEO, Health2Sync; and [Right] Claudio Longo, President, AstraZeneca Taiwan](/sites/mhn/files/styles/230x130/public/2025-07/%2528Left%2529%2520Ed%2520Deng%252C%2520Co-founder%2520and%2520CEO%2520of%2520Health2Sync%2520and%2520%2528Right%2529%2520Claudio%2520Longo%252C%2520President%2520of%2520AstraZeneca%2520Taiwan.jpg?itok=XO7M1UnC)